Earle H. Spaulding recognised a disinfection framework was required since all reusable medical devices cannot be sterilised.1
The Spaulding Classification met a key unmet need that today still forms the basis of international medical device disinfection guidelines.
The Spaulding Classification
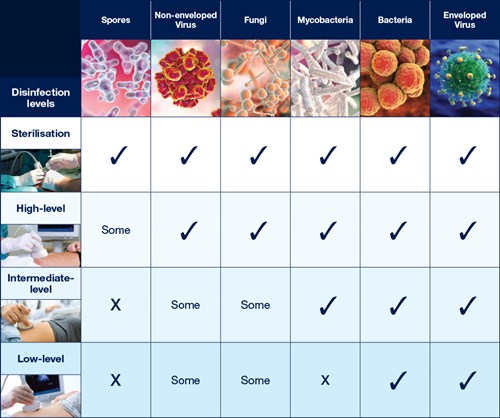
The Spaulding Classification stratifies the risk of infection transmission based on the patient tissue the device will contact during use. The device classification determines the level of disinfection/sterilisation required (Table 1).
Table 1: Overview of The Spaulding Classification.1-6
|
Spaulding Classification |
Medical Device Contacts |
Risk of Infection Transmission |
Disinfection Level |
|
Critical |
Sterile tissue or the bloodstream |
High |
Sterilisation* |
|
Semi-critical |
Mucous membranes or non-intact skin |
Medium |
High Level Disinfection (HLD) |
|
Non-critical |
Intact skin only |
Low |
Intermediate level (ILD) or Low level disinfection (LLD) |
*Critical ultrasound probes can be high level disinfected and used with a sterile sheath if sterilisation is not possible.4,5 In Scotland and Ireland, critical probes to guide needles or scan wounds are considered semi-critical.2,3
Sterilisation
Sterilisation destroys all microorganisms.42,3,5
Critical devices must be sterile when used.1
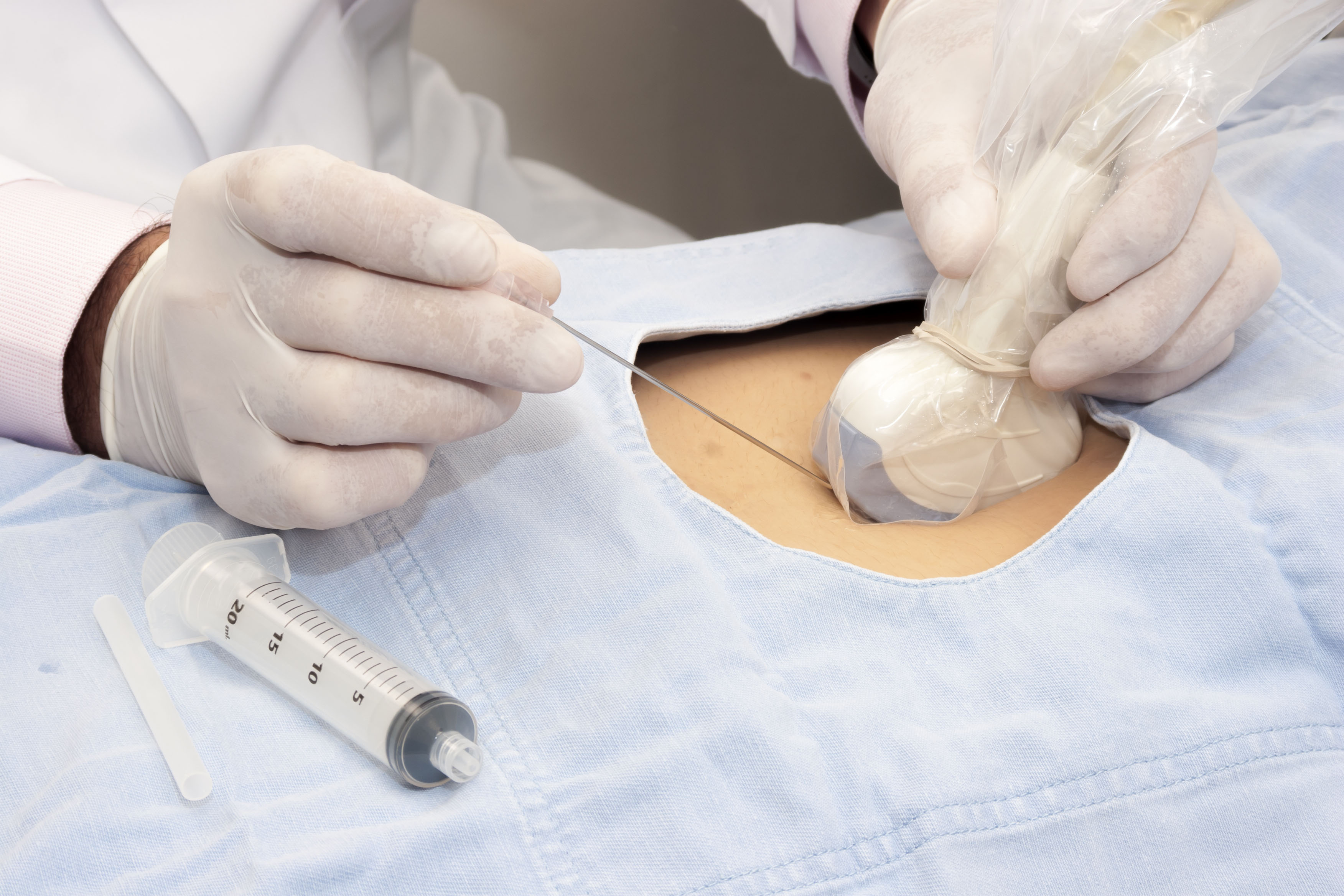
High Level Disinfection (HLD)
HLD destroys all microorganisms with the exception of high numbers of bacterial spores.4 A high level disinfectant is therefore bactericidal, virucidal (both lipid and non-lipid viruses), fungicidal and mycobactericidal.2,3,5
Semi-critical ultrasound probes must undergo HLD and be used with a sheath.2-5
Critical ultrasound probes that cannot be sterilised can also undergo HLD.4,5 They must also be used with a sterile sheath.4,5
Low & Intermediate Level Disinfection
Low level and intermediate level disinfection may be used for disinfection of non-critical probes contact with healthy, unbroken skin.5
Low level disinfection (LLD) is the elimination of most bacteria, some fungi and some viruses.5
Intermediate level disinfection is the elimination of most bacteria including mycobacteria, most fungi and some viruses but not bacterial spores.5
Understanding Disinfection
It is important to recognise there are differences between disinfection levels and sterilisation. Correctly applying The Spaulding Classification to medical devices is a key part of keeping patients safe from healthcare-associated infections (HAIs).
- Spaulding EH (1968). Chemical disinfection of medical and surgical materials. Disinfection, sterilization, and preservation. Lawrence C, Block SS. Philadelphia (PA), Lea & Febiger: 517-531.
- Health Protection Scotland (HPS), Health Facitlities Scotland (HFS), National Services Scotland (NHS) 2016. NHSScotland Guidance for Decontamination of Semi-Critical Ultrasound Probes; Semi-invasive and Non-invasive Ultrasound Probes. Version 1.0. March 2016.
- Health Service Executive (HSE) Quality Improvement Division 2017. HSE Guidance for Decontamination of Semi‐critical Ultrasound Probes; Semi‐invasive and Noninvasive Ultrasound Probes. Document: QPSD-GL-028-1.
- Society and College of Radiographers (SCoR) and British Medical Ultrasound Society (BMUS) 2019. "Guidelines For Professional Ultrasound Practice." Revision 3, December 2018.
- European Society of Radiology (ESR) 2017. Infection prevention and control in ultrasound - best practice recommendations from the European Society of Radiology Ultrasound Working Group.
- WHTM 01-06. 2014- Decontamination of flexible endoscopes Part C: Operational management, NHS Wales Shared Services Partnership – Specialist Estates Services: 74.
